Have you ever thought about what happens inside your body at the very smallest level? It’s pretty amazing, actually, how tiny living bits, like cells, deal with their surroundings. These little parts of us are always working, always reacting to the liquid they find themselves in. It’s a bit like us reacting to the weather outside, you know, whether it’s sunny or rainy, cells also respond to their immediate environment.
So, there are these three main kinds of liquid situations that cells might encounter, and each one makes them act in a different way. We're talking about hypertonic, isotonic, and hypotonic solutions here. Each of these situations tells a distinct story about how water moves around these tiny living structures. It’s all about balance, or the lack of it, really, when you get right down to it.
Understanding these different liquid environments helps us get a clearer picture of how our cells keep themselves going. It shows us, in a way, just how delicate and responsive these fundamental building blocks of life truly are. What happens to a cell when it's in one of these solutions can be pretty dramatic, sometimes leading to big changes in its shape or even its ability to stay intact.
- Westport Library
- Oregon Zoo Tickets
- Citizenm New York Times Square Hotel New York Ny
- Atlantic Station Movies
- Omni Atlanta Hotel At Centennial Park
Table of Contents
- What Makes Cells Change?
- How Does a Hypotonic Environment Affect Cells?
- What Happens in an Isotonic Setting?
- What About a Hypertonic Situation?
- Why Do Cells React Differently to Hypertonic vs Hypotonic Solutions?
- What Can We Learn from Hypertonic and Hypotonic Cell Behavior?
- A Closer Look at Water Movement
- What's the Big Deal with Water Moving In or Out?
What Makes Cells Change?
Cells, these tiny powerhouses, are always interacting with the liquid around them. This interaction, you know, determines their well-being. The way a cell behaves, whether it swells up or shrinks down, depends on how much stuff is dissolved in the liquid outside compared to what's inside the cell itself. It’s a constant give and take, almost like a silent conversation between the cell and its watery home. So, the terms hypertonic, isotonic, and hypotonic are simply ways to describe these different liquid surroundings.
When we talk about a cell changing, we are mostly talking about water moving. Water is a really important player here, as a matter of fact. It tends to move from areas where there's a lot of it to areas where there's less of it, trying to balance things out. This movement is what causes all the interesting reactions we observe in cells when they are put into different kinds of solutions. It’s a very natural process, you see, always seeking a kind of peaceful equality.
How Does a Hypotonic Environment Affect Cells?
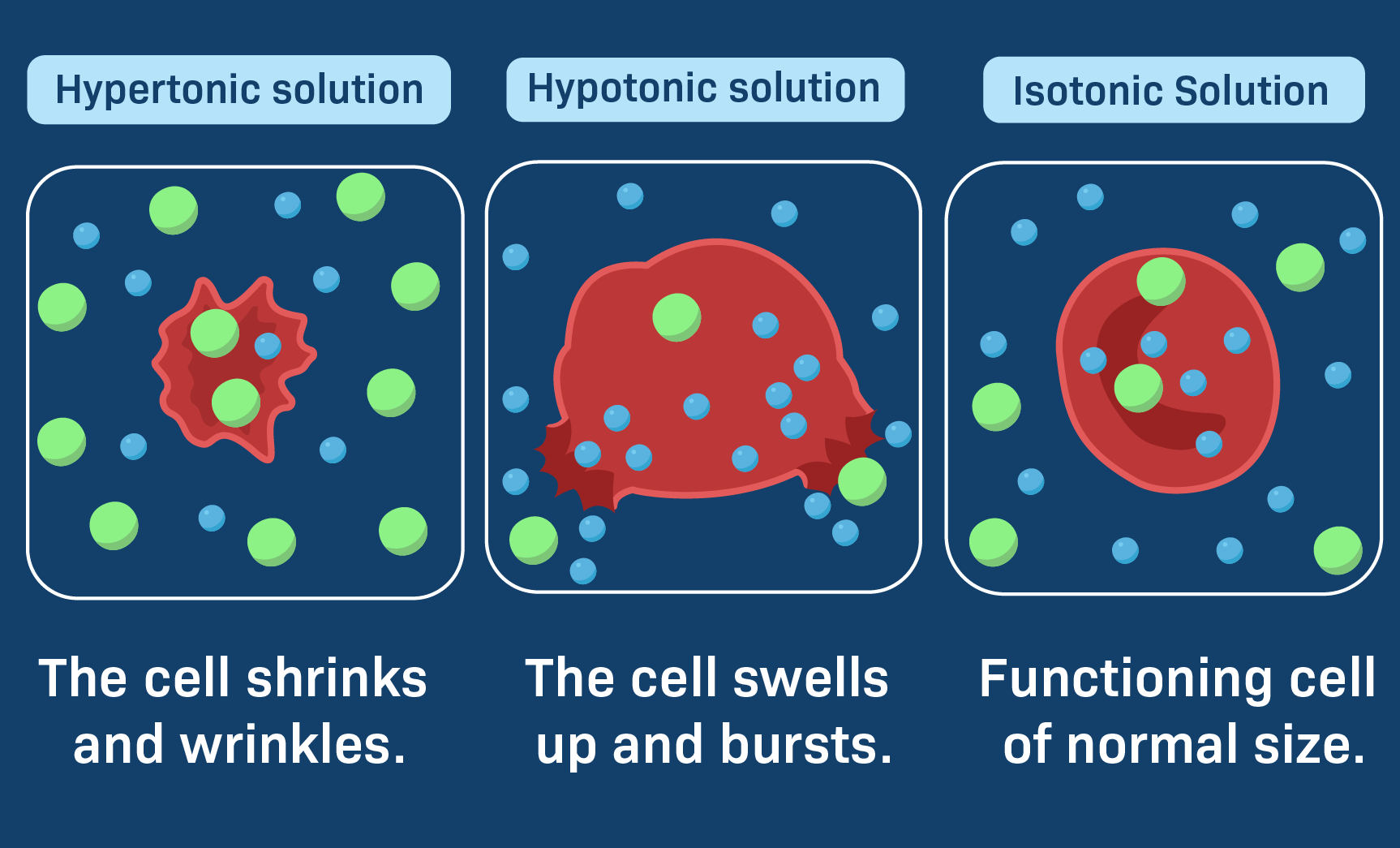
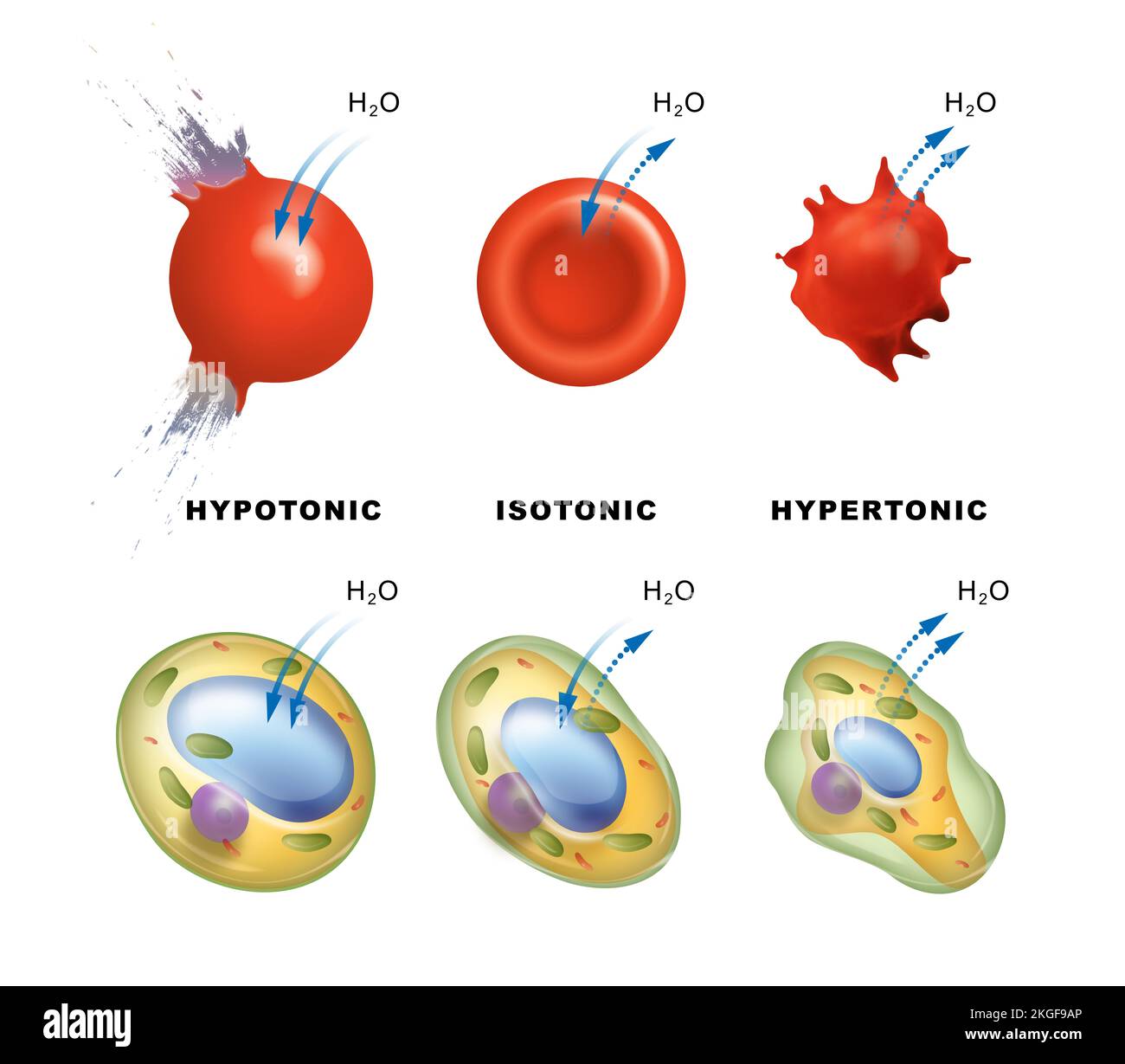
Imagine a cell sitting in a hypotonic solution. In this kind of liquid, there's a lot more water outside the cell than there is inside. So, what happens? Well, water, being the natural balancer it is, begins to rush into the cell. It’s like a sudden flood, really, pushing its way through the cell’s outer layer. This influx of water causes the cell to get bigger and bigger, sort of like a balloon filling up with air.
As more and more water pours in, the cell expands. It stretches, and the pressure inside builds up. This can become quite a problem for the cell, you know, as it has its limits. If too much water keeps coming in, the cell can reach a point where it simply can't hold any more. At that stage, the cell’s outer boundary might give way, causing it to burst open. It's a pretty dramatic end for a tiny cell, all because of too much water trying to get in.
The cell, in a way, becomes overwhelmed by its watery surroundings. It just keeps taking in fluid, unable to stop the flow. This situation highlights how delicate a cell’s structure can be, and how important it is for it to be in a balanced environment. So, when you hear about a hypotonic solution, think of a cell getting bigger and bigger, perhaps to the point of breaking apart.
What Happens in an Isotonic Setting?
Now, let's think about a different kind of liquid situation: an isotonic solution. This is, arguably, the most comfortable place for a cell to be. In an isotonic liquid, the amount of dissolved stuff outside the cell is just right, it’s the same as the amount of dissolved stuff inside the cell. Because of this perfect match, water moves into the cell at the same speed that it moves out.
There's no big rush of water in one direction or the other. It’s a truly balanced exchange, you know, a sort of peaceful dance. The cell stays its usual size, neither getting bigger nor smaller. It’s a state of equilibrium, where everything is just so. This is the kind of environment our body usually tries to keep for its cells, ensuring they can carry out their jobs without any stress from too much or too little water.
So, if you imagine a cell that’s perfectly content, not swelling up or shriveling, that’s a cell in an isotonic solution. It’s pretty much ideal, allowing the cell to maintain its proper shape and function without any fuss. There’s simply no net movement of water, meaning the amount going in equals the amount coming out, keeping things steady.
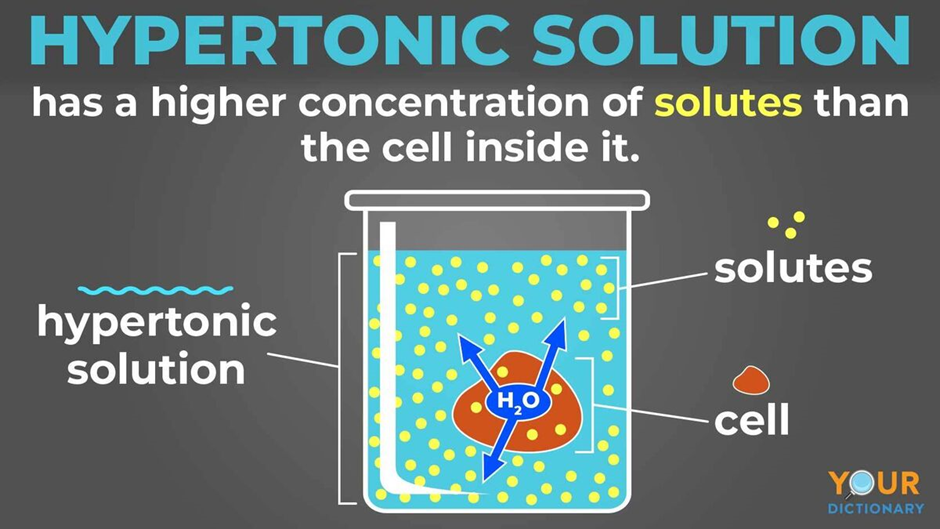
What About a Hypertonic Situation?
Let's turn our attention to the hypertonic solution. This is quite the opposite of the hypotonic one. In a hypertonic liquid, there's actually more dissolved stuff outside the cell than there is inside it. This means there's less free water outside the cell compared to the inside. So, what does water do in this scenario? It starts to leave the cell.
Water, trying to balance things out, moves from inside the cell, where there's a higher concentration of water, to the outside, where there's less. It's like the cell is drying out, you know, slowly losing its precious fluid. As water exits the cell, the cell begins to shrink. It loses its plumpness and starts to shrivel up, becoming smaller and smaller.
This loss of water can be very damaging to a cell. If too much water leaves, the cell can become so shrunken that it can no longer work properly. It might even collapse in on itself, becoming inactive. So, when a cell is placed in a hypertonic solution, the primary event is water moving out, leading to the cell getting smaller and losing its normal form.
Why Do Cells React Differently to Hypertonic vs Hypotonic Solutions?
The reason cells react so differently in hypertonic versus hypotonic solutions comes down to that basic principle of water movement. Water always wants to move from a place where it is more concentrated to a place where it is less concentrated. This is a very fundamental aspect of how liquids behave, really.
In a hypotonic setting, there's a higher concentration of water outside the cell. This means water naturally wants to move into the cell to balance things out. Conversely, in a hypertonic setting, the concentration of water is lower outside the cell compared to inside. So, water exits the cell, again, trying to achieve a kind of balance. It's all about trying to make the water concentration equal on both sides of the cell's outer boundary, which, you know, is a pretty simple yet powerful idea.
The cell's reaction is simply its way of responding to these different water flows. It doesn't have a choice, in a way; it's a physical process driven by the natural tendency of water to spread out evenly. This distinction in water movement is the core reason for the very different outcomes observed in cells when they face hypotonic or hypertonic environments.
What Can We Learn from Hypertonic and Hypotonic Cell Behavior?
Looking at how cells respond to hypertonic and hypotonic solutions teaches us a lot about the importance of maintaining a stable internal environment. For living things, keeping cells in an isotonic state is, well, pretty much essential for survival. Our bodies have ways to make sure our cells are always bathed in a liquid that keeps them happy and working as they should.
This understanding helps us appreciate the delicate balance within our own bodies. It also has practical uses, for example, in medical treatments or in how we preserve certain biological materials. Knowing how cells react to different water concentrations means we can predict what might happen and take steps to protect them. It's a simple concept, but it has, arguably, far-reaching implications for how we understand life itself.
So, the next time you hear about hypertonic or hypotonic, remember it’s all about water finding its way, and how that movement shapes the tiny, vital components that make up every living thing. It truly shows the precision needed for life to thrive, right down to the smallest parts.
A Closer Look at Water Movement
The idea that water just "moves" might seem a bit simple, but it's a really important process for cells. This movement is always happening, even when we can't see it. It's a constant, gentle flow, or sometimes a rapid rush, depending on the situation. The cell's outer covering, its membrane, is somewhat selective about what gets in and out, but water, for the most part, can pass through it quite easily.
So, when we talk about water rushing in or out, we are describing this constant attempt by the water molecules to achieve an even spread. If there are lots of dissolved bits on one side, those bits take up space, meaning there’s less water. Water from the side with more pure water will then try to dilute the side with more dissolved bits. It’s a pretty natural drive, you know, for balance.
What's the Big Deal with Water Moving In or Out?
You might wonder, why does it matter if a cell gets a little bigger or a little smaller? Well, for a cell, its shape and its internal contents are incredibly important for doing its job. If a cell swells too much, like in a hypotonic solution, its outer layer can stretch past its limit and simply break. Once that happens, the cell can no longer function; its insides spill out, and it's essentially done.
On the other hand, if a cell loses too much water, as it does in a hypertonic solution, it shrivels. This loss of water means its internal machinery, the bits that make it work, can’t operate properly. They become too crowded or too dry to do what they are supposed to do. So, you know, even a slight change in water balance can have a very significant impact on a cell’s ability to survive and carry out its vital tasks.
The big deal is that maintaining the right water balance is, basically, critical for cell health and, by extension, for the health of the entire living thing. Cells are quite sensitive to these changes, and their reactions to hypertonic, isotonic, and hypotonic solutions show just how finely tuned their existence really is. It's a matter of life or death for these tiny structures, honestly.
This article explored how cells react differently in hypotonic, isotonic, and hypertonic solutions. It covered how water rushes into a cell in a hypotonic solution, potentially causing it to expand or burst. It also explained that in an isotonic solution, there is no net flow of water, keeping the cell stable. Furthermore, it detailed how water moves out of a cell in a hypertonic solution, leading to shrinkage. The discussion highlighted that these three terms describe whether water will move into or out of a cell, and the impact this has on cell integrity and function.
Related Resources:
Detail Author:
- Name : Carolina Schmidt
- Username : ashlee.lesch
- Email : lucious51@hotmail.com
- Birthdate : 2001-08-31
- Address : 37265 Robel Forest New Rene, NY 67081-9029
- Phone : (432) 316-6211
- Company : Hamill, Schmeler and Reilly
- Job : Mechanical Equipment Sales Representative
- Bio : Vel quo accusantium mollitia repellendus. Eum quod qui rerum et doloremque. Temporibus voluptas rerum repellat cum quisquam. Beatae quae hic ab eveniet et magni vel magni.
Socials
twitter:
- url : https://twitter.com/solon_real
- username : solon_real
- bio : Non veniam ut maxime. Numquam est recusandae quia et. Accusamus aut sit architecto sit culpa ducimus ea non.
- followers : 2809
- following : 887
tiktok:
- url : https://tiktok.com/@solon_id
- username : solon_id
- bio : Veniam rerum ut omnis. Pariatur provident non et sunt iusto dignissimos dolore.
- followers : 4099
- following : 1740
facebook:
- url : https://facebook.com/solon.schimmel
- username : solon.schimmel
- bio : Tempore id impedit ipsum tenetur amet minima cupiditate. Sint id pariatur amet.
- followers : 3334
- following : 2160
linkedin:
- url : https://linkedin.com/in/solon6866
- username : solon6866
- bio : Voluptatem aut distinctio quod illo ut natus.
- followers : 1218
- following : 2800
instagram:
- url : https://instagram.com/sschimmel
- username : sschimmel
- bio : Laborum et a minus recusandae aut. Molestias ut et explicabo nihil facilis fugiat.
- followers : 1153
- following : 1792